Details of the Drug
General Information of Drug (ID: DM57SVW)
| Drug Name |
benzamil
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Benzamil; 2898-76-2; UNII-04659UUJ94; N-(N-Benzylamidino)-3,5-diamino-6-chloropyrazine carboxamide; GNF-Pf-192; CHEMBL212579; CHEBI:34558; 04659UUJ94; 3,5-Diamino-6-chloro-N-(imino((phenylmethyl)amino)methyl)pyrazinecarboxamide; 3,5-diamino-N-(N'-benzylcarbamimidoyl)-6-chloropyrazine-2-carboxamide; Pyrazinecarboxamide, 3,5-diamino-6-chloro-N-(imino((phenylmethyl)amino)methyl)-; Spectrum3_001823; Prestwick1_000657; Prestwick3_000657; Prestwick2_000657; Prestwick0_000657; Lopac-B-2417; AC1L33CQ; Lopac0_000211; KBioGR_000300
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
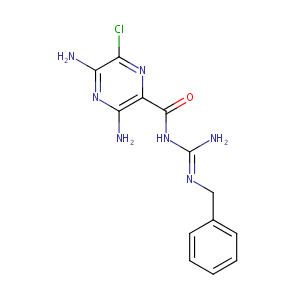 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 319.75 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
References
